Clinical Pharmacology of Local Anesthetics
Highlights
- Local anesthetics work by binding to the α subunit of the voltage-gated Na+ channels, thus preventing the generation and conduction of nerve impulses (View Highlight)
- Cocaine, the archetypical ester, is the only naturally occurring LA. (View Highlight)
- The introduction of the amide LA lidocaine in 1948 was transformative. (View Highlight)
- Ropivacaine and levobupivacaine are the only commercially available single-enantiomer (single-optical-isomer) LAs. Both are S(–)-enantiomers, avoiding the increased cardiac toxicity associated with racemic mixtures and the R(+)-isomers (View Highlight)
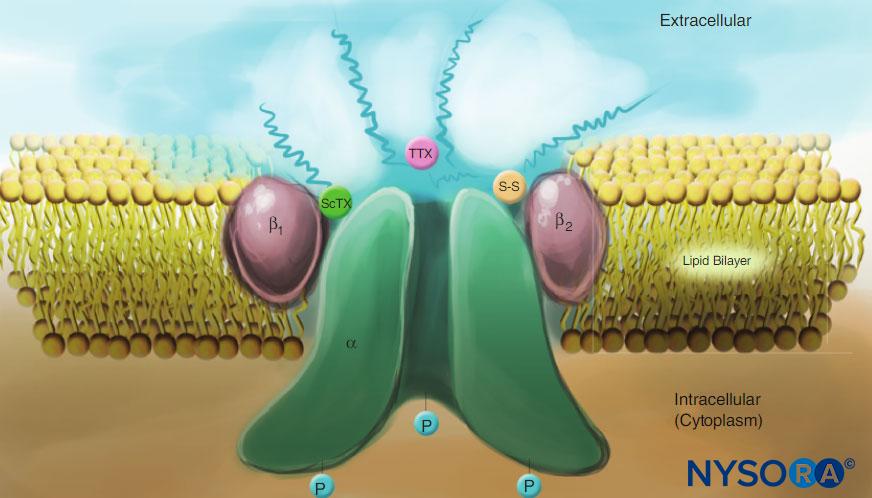
- The α-subunit, the site of ion conduction and LA binding, has four homologous domains, each with six α-helical membrane-spanning segments (View Highlight)
- The external surface of the α-subunit is heavily glycosylated, which serves to orient the channel properly within the plasma membrane (View Highlight)
- In contrast to local anesthetics, note that both scorpion toxins (ScTX) and tetrodotoxin (TTX) have binding sites on the external surface of the channel (View Highlight)
- In clinical practice, LAs are typically described by their potency, duration of action, speed of onset, and tendency for differential sensory nerve block (View Highlight)
- Esters undergo rapid hydrolysis in blood, catalyzed by non-specific esterases (View Highlight)
- Procaine and benzocaine are metabolized to para-aminobenzoic acid (PABA), the species underlying anaphylaxis to these agents. (View Highlight)
- There is also contro-versy about transient neurologic symptoms and persistent sacral deficits after lidocaine spinal anesthesia. The reports and the controversy have persuaded many physicians to abandon lidocaine spinal anesthesia (View Highlight)
